Research Themes
Our lab is mainly interested in (1) a microfluidic/nanofluidic device, essentially a network of micro/nanoscale fluidic components, and (2) application of microfluidic and nanofluidic device to analyze biomolecules such as proteins, peptide, RNA, and DNA, which are crucial in assessing physiological state of a human. We are also working on (3) individual microscale fluidic components including micropump, microvalve, micromixer, and microscale dialyzer, (4) novel microfabrication techniques such as micro 3D printing, laser machining, and hot embossing for plastic-based microfluidic/nanofluidic devices. Lastly, we are keen to explore (5) interesting micro/nanoscale physicochemical phenomena that are not observable in conventional macroscale fluidic systems including electrokinetics and microscale heat transfer. Ultimately, we are pursuing microanalytical systems or lab-on-a-chip (LOC) that can determine clinically and biologically essential biomolecules accurately, rapidly, and reliably - so that they can replace bulky, expensive, material-consuming, but essential analytical instruments in research or clinical laboratory.
1. Novel Polymer Microfabrication Techniques
1.1. High-precision Laser Machining for Polymer Microfabrication
In order to fabricate a microfluidic device using hard plastic such as PMMA (polymethyl methacrylate)or PC (polycarbonate), hot embossing was used to replicate microfluidic features made on
a master mold. However, the master mold is usually made using cleanroom-based microfabrication techniques which is costly and slow. Using optimized CO2 laser cutting on a thin plastic sheet,
sub-50 μm microfluidic features was patterned directly from a CAD design less than 10 min. (Published in KSPE 2017, KSPE 2018, BioChip 2018, and IEEE MEMS 2019 conferences).
1.2. Advanced PMMA Chip Bonding
The cut-through patterned PMMA sheet has to be bonded to PMMA coverslips to enclose microfluidic features. Traditionally, these PMMA layers are thermally bonded using a
hot-embossing machine or hot press after thoroughly cleaned. However, even small dusts and debris from laser cutting can cause
an unbonded area, leading to a incomplete bonding.
Thus, we employ a simple, fast, and reliable tape-based bonding technique TPLMPT (tape-liner-supported plastic laser micromachining and pattern transfer). We also employ solvent-assisted
thermal bonding in conjunction without adhesives (Published in KSPE 2018, BioChip 2018 and MEMS 2019 conferences, and Sensors and Actuators A, Sensors and Actuators B Journals).
1.3. 3D printed microfluidics
The 3D printing techniques have been revolutionizing almost all branches of
the manufacturing industry in terms of speed, customizability, and scalability. The 3D
Printing also poses tremendous impacts on the microfluidics field because it breaks the barrier of inherent geometrical limitation of planar microfluidic
devices (2D or 2.5D) to build a 3D device enabling an unprecedented improvement of its functionality. Recently, many research groups have been working toward
printing a microfluidic device using SL (stereolithography) printers with PMMA (hard, transparent hard plastic)- or PDMS (flexible transparent polymer)-like
material properties.
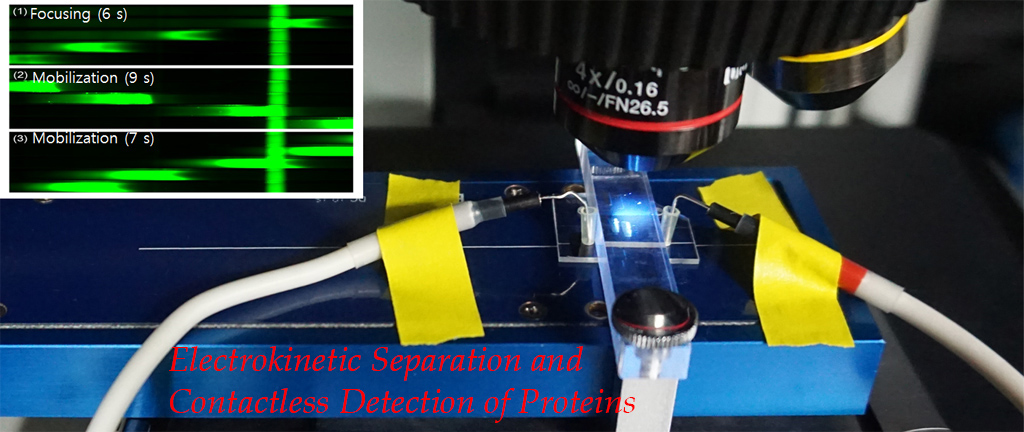
2. Electrokinetic Separation and Detection of Biomolecules
2.1. Microfluidic Isoelectric Focusing for Protein Detection
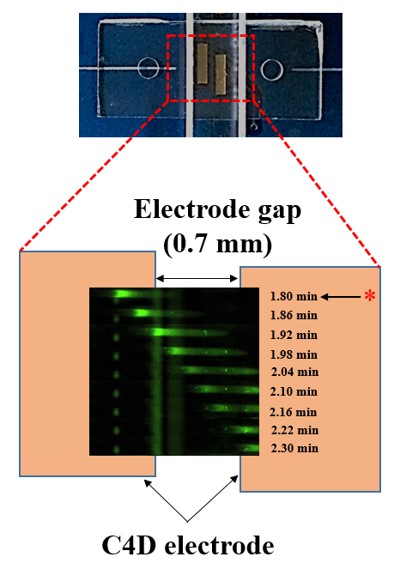 Microfluidic IEF (isoelectric focusing), regardless of advantages over conventional capillary format, relies on a bulky and expensive optical detection system. To
overcome this drawback, we opt for conductivity-based protein detection. Numerical analysis based
on a model protein, green fluorescence protein (GFP), indicates that focusing of protein induces detectable
conductivity change. Experimental results show that GFP peaks can be successfully detected
using microfluidic IEF integrated with C4D. Our novel conductivity assay could be a viable alternative
to optical methods when the size and portability of the overall detection system are critical (Published in BioChip 2015, MicroTAS 2016, MicroTAS 2018
conferences).
Microfluidic IEF (isoelectric focusing), regardless of advantages over conventional capillary format, relies on a bulky and expensive optical detection system. To
overcome this drawback, we opt for conductivity-based protein detection. Numerical analysis based
on a model protein, green fluorescence protein (GFP), indicates that focusing of protein induces detectable
conductivity change. Experimental results show that GFP peaks can be successfully detected
using microfluidic IEF integrated with C4D. Our novel conductivity assay could be a viable alternative
to optical methods when the size and portability of the overall detection system are critical (Published in BioChip 2015, MicroTAS 2016, MicroTAS 2018
conferences).
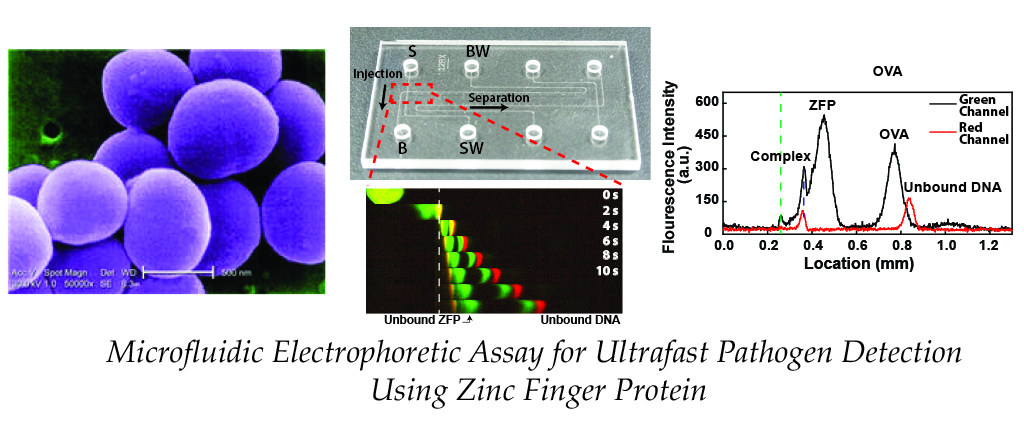
2.2. Denaturation-free EMSA Detection of double-stranded DNA
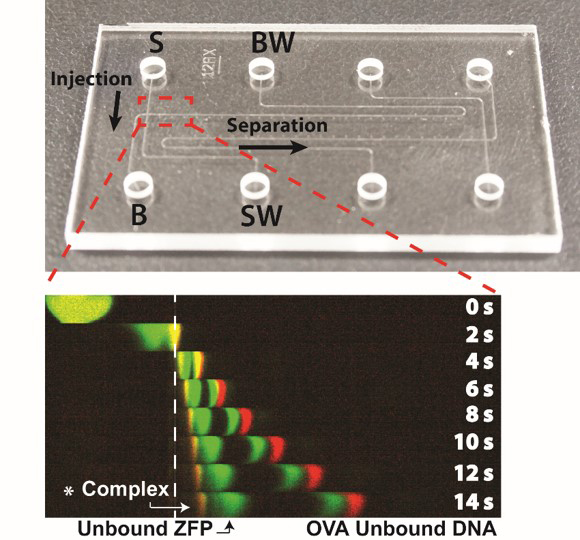 We reported microfluidic homogeneous electrophoretic affinity (MHEA) assay for DNA
quantification using zinc finger protein (ZFP) and polyacrylamide-gel (PAG) sieving matrix. Using the MHEA
assay with ZFP, a nucleic-acid-binding probe, the double-stranded (dsDNA) can be directly detected without the
requirement for a time-consuming DNA hybridization process. To demonstrate the performance of our assay, seb
gene (Staphylococcal enterotoxin B), an important bacterial biomarker for food poisoning is analyzed.
Experimental results show that the pathogen-specific dsDNA is successfully quantified (2~500 nM) with a short
separation distance (197 μm) and time (14 s) facilitated by the polyacrylamide resolving gel (published in MicroTAS 2017)
conference).
We reported microfluidic homogeneous electrophoretic affinity (MHEA) assay for DNA
quantification using zinc finger protein (ZFP) and polyacrylamide-gel (PAG) sieving matrix. Using the MHEA
assay with ZFP, a nucleic-acid-binding probe, the double-stranded (dsDNA) can be directly detected without the
requirement for a time-consuming DNA hybridization process. To demonstrate the performance of our assay, seb
gene (Staphylococcal enterotoxin B), an important bacterial biomarker for food poisoning is analyzed.
Experimental results show that the pathogen-specific dsDNA is successfully quantified (2~500 nM) with a short
separation distance (197 μm) and time (14 s) facilitated by the polyacrylamide resolving gel (published in MicroTAS 2017)
conference).
3. MEMS (Micro-Electro-Mechanical-Systems) for Integrated Microfluidics
3.1. PCB-based Electrolytic Micropump
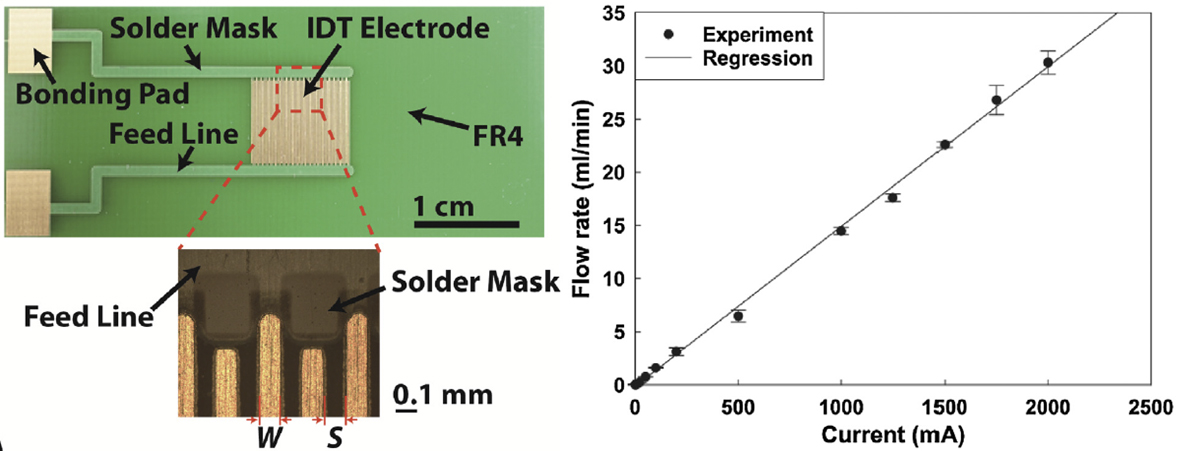 We developed an electrolytic micropump based on an electrode chip fabricated on a printed circuit board (PCB).
Gold interdigitated (IDT) electrodes are patterned on a PCB to minimize ohmic loss during electrolysis.
As predicted by the theory of water electrolysis, the micropump produces flow rate increasing linearly with current at a wide range (1 mA–2 A).
Our micropump yields the maximum flow rate of 31.6 ml/min and maximum backpressure of 547 kPa (at 34 μml/min), significantly high compared with the previous micropumps based on various actuation mechanisms including piezoelectric actuation, electroosmosis and phase change.
We anticipate the PCB-based electrolysis pump will be used in portable lab-on-a-chip devices where an integrated microscale pressure source with low power consumption and simple fabrication is crucial
(published in KSME 2016, KSPE 2017, KSPE 2018, MicroTAS 2017
and MicroTAS 2018 conferences, and Sensors and Actuators A: Physical 2015).
We developed an electrolytic micropump based on an electrode chip fabricated on a printed circuit board (PCB).
Gold interdigitated (IDT) electrodes are patterned on a PCB to minimize ohmic loss during electrolysis.
As predicted by the theory of water electrolysis, the micropump produces flow rate increasing linearly with current at a wide range (1 mA–2 A).
Our micropump yields the maximum flow rate of 31.6 ml/min and maximum backpressure of 547 kPa (at 34 μml/min), significantly high compared with the previous micropumps based on various actuation mechanisms including piezoelectric actuation, electroosmosis and phase change.
We anticipate the PCB-based electrolysis pump will be used in portable lab-on-a-chip devices where an integrated microscale pressure source with low power consumption and simple fabrication is crucial
(published in KSME 2016, KSPE 2017, KSPE 2018, MicroTAS 2017
and MicroTAS 2018 conferences, and Sensors and Actuators A: Physical 2015).
3.2. Cavitation-microstreaming Micromixer
 We advance a microfluidic mixer based on cavitation microstreaming by optimizing performance using highspeed
flow visualization. Using transfer tapes and experimentally optimized laser machining, a micromixer is
microfabricated and assembled with ease and accuracy. For mixing, bubbles trapped in air pockets are excited at
resonance frequencies using a piezoelectric actuator. The resonance frequencies of the micromixer are found using
electrical impedance spectroscopy and high-speed images generated by seeded microbeads around the
vibrating bubbles in the mixing chamber (published in KSPE 2017, KSPE 2018, and BioChip 2018
conferences).
We advance a microfluidic mixer based on cavitation microstreaming by optimizing performance using highspeed
flow visualization. Using transfer tapes and experimentally optimized laser machining, a micromixer is
microfabricated and assembled with ease and accuracy. For mixing, bubbles trapped in air pockets are excited at
resonance frequencies using a piezoelectric actuator. The resonance frequencies of the micromixer are found using
electrical impedance spectroscopy and high-speed images generated by seeded microbeads around the
vibrating bubbles in the mixing chamber (published in KSPE 2017, KSPE 2018, and BioChip 2018
conferences).
4. Study of Micro/nanoscale Physicochemical Phenomena
4.1. Microfluidic Evaporation Cooling
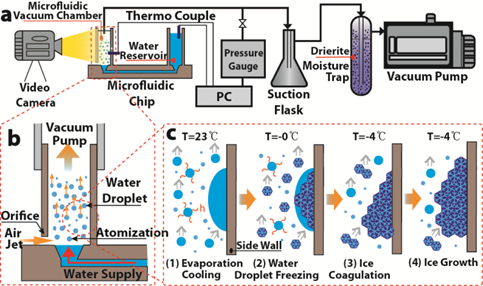 We developed a simple water-based evaporative cooling integrated into a microfluidic chip for
temperature control and freezing of biological solution. Aqueous solutions are atomized in our device and evaporation of microdroplets under vacuum
removes heat effectively. We achieve rapid cooling (−5.1 ◦C/s) and low freezing temperature
(−14.1 ◦C). Using this approach, the freezing of deionized water and protein (BSA) solution was demonstrated.
This simple, yet effective cooling device may improve many microfluidic applications currently
relying on external power-hungry instruments for cooling and freezing (published in
KSME 2014, BioChip 2014 and MicroTAS 2014 conferences, and Review of Scientific Instrument 2015).
We developed a simple water-based evaporative cooling integrated into a microfluidic chip for
temperature control and freezing of biological solution. Aqueous solutions are atomized in our device and evaporation of microdroplets under vacuum
removes heat effectively. We achieve rapid cooling (−5.1 ◦C/s) and low freezing temperature
(−14.1 ◦C). Using this approach, the freezing of deionized water and protein (BSA) solution was demonstrated.
This simple, yet effective cooling device may improve many microfluidic applications currently
relying on external power-hungry instruments for cooling and freezing (published in
KSME 2014, BioChip 2014 and MicroTAS 2014 conferences, and Review of Scientific Instrument 2015).